America
After Cough Syrup, Now Indian-Made Eye Drops Cause Concern; CDC Flags EzriCare
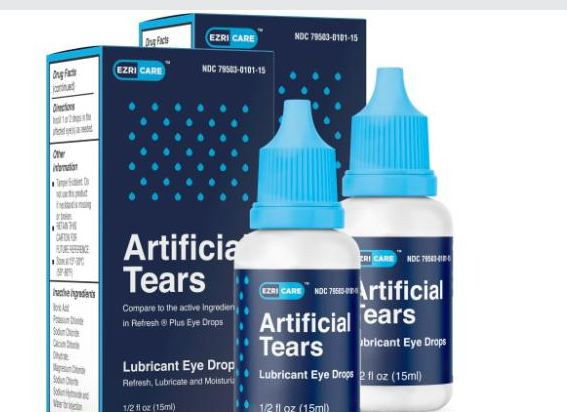
ATLANTA, GA: The US Center for Disease Control and Prevention (CDC) has flagged concern over the use of an Indian eye drop that is causing death and blindness.
The CDC has, in recent months, traced three deaths, eight cases of blindness and dozens of infections caused by a highly drug-resistant bacteria linked to EzriCare artificial tears, made by Global Pharma Healthcare, The New York Times reported.
Drug-resistant bacteria Pseudomonas aeruginosa has been a cause of concern among people with weak immune systems, residents of nursing homes and patients using catheters and breathing tubes.
In February, Global Pharma Healthcare recalled and stopped the production of both EzriCare Artificial Tears and Delsam Pharma’s Artificial Tears. And the US Food and Drug Administration (FDA) had instructed doctors and consumers not to purchase the product from the market and warned those who have already purchased it not to use it.
The warning issued by the FDA read, “using contaminated artificial tears increases the risk of eye infections that could result in blindness and deathâ€.
However, the CDC said it was concerned that the bacteria could gain a foothold in the US, as the bacteria showed signs of spreading among asymptomatic patients, in a Connecticut care center, who had the bacteria colonized in their bodies. Such spread tends to occur when patients touch common items or when healthcare workers transmit the germs.
According to experts, pseudomonas is very hard to get rid of. Pseudomonas is especially difficult to eradicate, both from health care facilities, where it clings tenaciously to sink drains, water faucets and other moist environments, and from patients who develop bloodstream infectionsâ€, David van Duin, an infectious disease specialist at the University of North Carolina School of Medicine, was quoted as saying.
After the FDA had issued the warning in February, a group of drug inspectors from the Central and Tamil Nadu governments conducted an inspection at the company premises which is 40 km south of Chennai.
While refusing to comment on the FDA’s remarks on the eyedrops, the Tamil Nadu drug regulator now said it found “no contamination†in samples of eye drops manufactured by Global Pharma, media reports said.
This is the third such incident where an Indian pharmaceutical product has come under scrutiny.
Last year, dozens of deaths were reported among children in Gambia and Uzbekistan due to Indian cough syrups.
Cough and cold syrups manufactured by New Delhi-based Maiden Pharmaceuticals were linked to the deaths of at least 70 children in Gambia, while Delhi-based Marion Biotech’s cough syrups were linked to the deaths of 18 children in Uzbekistan.

7 hours ago
PM Modi moots IBSA Fund for climate resilient agriculture at Johannesburg meet

7 hours ago
Piyush Goyal conveys PM Modi's wishes to Netanyahu, highlights progress in trade dialogue

7 hours ago
INS Sahyadri, Australian Navy's HMAS Ballarat participate in 'AUSINDEX' in Northern Pacific

7 hours ago
PM Modi meets his Japanese counterpart; bilateral cooperation discussed

7 hours ago
Lee vows to host G20 summit in 2028 with 'profound sense of responsibility'

7 hours ago
PM Modi, South African Prez Ramaphosa push for enhanced trade, tech and Global South voice

15 hours ago
G20 Johannesburg summit calls for improving global governance

15 hours ago
EAM Jaishankar speaks to Ukrainian FM, discusses latest conflict-related developments

15 hours ago
PM Modi holds significant discussions with world leaders during Jo'burg G20 Summit

15 hours ago
US says Russia-Ukraine peace draft made with Moscow's 'input'

15 hours ago
Ukraine, US to hold consultations on peace plan in Switzerland

15 hours ago
Houthi court sentences 18 Yemeni UN aid workers to death for 'spying for Israel'

15 hours ago
Justice Surya Kant to take oath as 53rd CJI tomorrow






















